News

Prof. Dorota Gryko and Prof. Bartosz Grzybowski are winners of the Prof. Wojciech Świętosławski
Loading...
Loading error

Dr. Kajetan Dąbrowa, Assoc. Prof. Rafał Loska and Dr. Michał Ociepa are winners of NCN grants
Loading...
Loading error
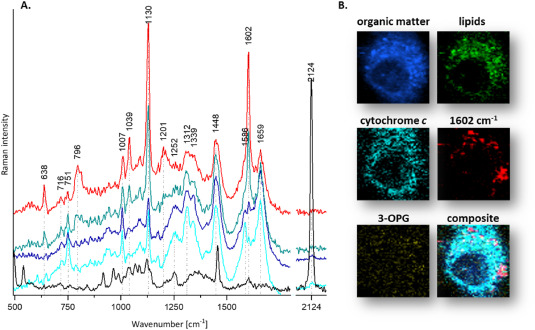
Article by Prof. Jacek Młynarski and Prof. Małgorzata Barańska in Biosensors and Bioelectronics
Loading...
Loading error

Dr. Marek Grzybowski and Assoc. Prof. Bartosz Zambroń are the winners of SONATA BIS
Loading...
Loading error
Loading...
Loading error
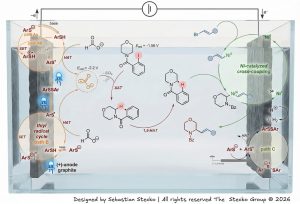
Siddharth K. Dave and Sebastian Stecko have just reported a general method for photoelectrochemical site-selective α- and β-C(sp3)−H alkenylation of amines with vinyl bromides which was published in Chemical Science journal (doi: 10.1039/D5SC08559D). In their protocol, regioselective activation of inert C–H bonds is achieved by an intramolecular hydrogen atom abstraction (HAT) by an oxidatively generated aryl radical. Depending on the HAT directing group attached to the amines’ N-atom, either 1,5- or 1,6-H-atom transposition occurred, leading to regioisomeric carbon-centered radical species. C-radicals thus formed at the α/β-position of the amines’ functionality undergo radical cross-coupling with Ni complex-activated vinyl bromide to provide the corresponding α- or β-functionalized amines. Good functional group tolerance, gram-scale experiments, post-functionalization, and demonstration of preparation of key structural scaffolds of the selected naturally occurring compounds and drug candidates greatly highlight the potential applicability of the presented method. The mechanistic experiments demonstrated that the reported protocol consists of a photoelectrochemically induced thiyl radical anodic cycle and an electrochemically driven Ni-catalytic cathodic cycle. The first one is responsible for the generation of CO2˙−, a XAT reagent capable of activation of aryl halide to provide aryl radical species. Cathodic reduction of a Ni(I) intermediate to the Ni(0) complex allows closing the Ni-catalyzed cycle, enabling the execution of the cross-coupling stage leading to a C–H functionalized amine derivative. The developed conditions enable precise synchronization of anodic and cathodic catalytic cycles occurring in the presented variant of paired electrolysis, which has been a significant challenge until now.
S. K. Dave, S. Stecko, „Remote α-and β-C(sp 3 )-H Alkenylation of Amines via Visible-Light Supported Paired Electrolysis”, Chem. Sci.