News
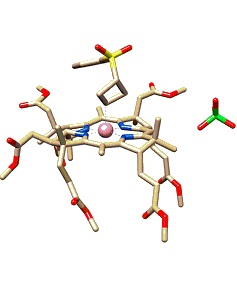
The publication by Prof. Daniel Gryko in the Journal of the American Chemical Society
Loading...
Loading error

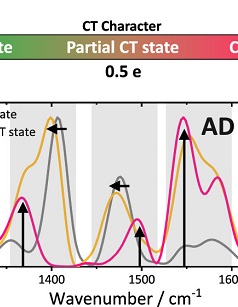
Article by Prof. Jacek Młynarski and Prof. Bartosz Grzybowski honored by ACS and RSC
Loading...
Loading error
Loading...
Loading error
The National Science Centre has announced the results of the SONATA BIS competition, dedicated to research projects aimed at establishing a new research team conducting basic science research. Funding was awarded to:
Dr. Anna Żądło-Dobrowolska for the project: „Targeting MMP-9 in Cancer Treatment: Computational Design and Experimental Validation of Potent, Safe Antitumor Agents”;
Dr. Michał Ociepa for the project: „Unlocking Access to New Bioisosteric Motifs: Radical Reactions and Reagents for the Incorporation of NCF3 and NCF2H Functional Groups”;
Assoc. Prof. Mykhaylo Potopnyk for the project: „Red and near-Infrared boron-containing TADF emitters for advanced optoelectronic and bioimaging applications”.
Congratulations and we wish you much success in your research!