An article by Wojciech Chaładaj Group in ACS Catalysis
The publication describes a new three-component reaction allowing the synthesis of α,β-unsaturated esters substituted with fluoroalkyl groups directly from alkynes, fluoroalkyl iodides and aryl formates. An important part of the work is devoted to in-depth mechanistic investigations revealing that the tandem process is composed of radical iodoperfluoroalkylation involving Pd(0)/Pd(I) chemistry and subsequent aryloxycarbonylation proceeding through Pd(0)/Pd(II) catalytic cycle employing carbon monoxide and phenoxide formed through off-cycle, base-induced decomposition of aryl formate. The developed conditions allow the reaction to be carried out without an external source of toxic carbon monoxide.
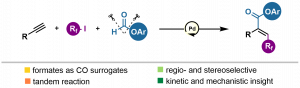
Article: https://pubs.acs.org/doi/10.1021/acscatal.1c00671
The research was carried out within the OPUS 11 grant from the National Science Center (2016/21/B/ST5/03178) using resources provided by Wroclaw Centre for Networking and Supercomputing (grant no. 518).