New publication of the Daniel Gryko group in Chemical Science
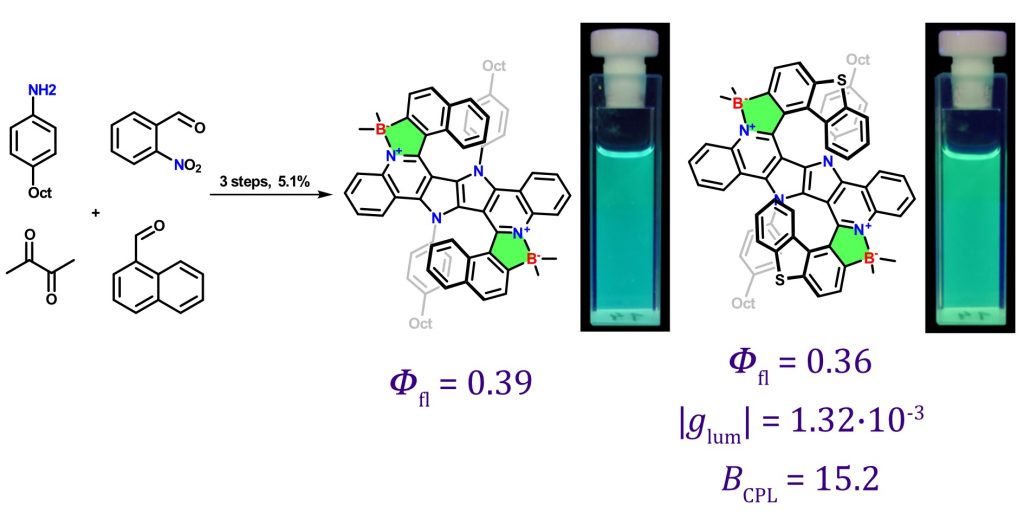
Prof. Daniel Gryko together with co-workers from Warsaw University, France and Italy discovered that it is possible, in just four steps, to transform 2-nitrobenzaldehyde into centrosymmetric, quadrupolar N,B-doped nanographenes possessing two nitrogen-boron dative bonds. A convergent fragment coupling strategy allowed rapid access to key intermediates bearing the 1,4-dihydropyrrolo[3,2-b]pyrrole core. This synthetic strategy can be extended to encompass double helicenes possessing two [7]helicene units bearing four five-membered rings. Interestingly only two enantiomers and not meso form are formed in the latter case. The obtained double helicene containing 14 fused rings, exhibits green emission characterized by reasonable circularly polarized luminescence brightness (BCPL) of about 15 M−1cm−1. The first author of the aper is Wojciech Petrykowski.
W.D. Petrykowski, N. Vanthuyne, C. Naim, F. Bertocchi, Y.M. Poronik, A. Ciesielski, M.K. Cyrański, F. Terenziani, D. Jacquemin, D.T. Gryko
“Double helicene possessing B–N dative bonds built on 1,4-dihydropyrrolo[3,2-b]pyrrole core” Chem. Sci., 2025
Congratulations and we wish you continued success!