Dr. Cina-Foroutan Nejad has published article in the Nature Communications
The possibility of forming π-bonds without a σ-bond has been a fascinating topic in chemistry. Several examples of such species have been described by theoretical chemists, namely, a unique Ti-C bond with no σ-donation from CO to Ti but a back-donation from Ti to π* of CO (10.1039/C0CP01519A).
In recent years several groups tried to synthesize organic molecules with π-bonds without a σ-bond. The general strategy to do so has been synthesizing stable 1,3- diradicals, where a π-bond between two remotely semi-occupied atomic p-orbitals may form but the σ-bond is ideally not expected. Is this strategy successful? In the present contribution using a combination of MO theory and topological analysis and I studied a recent molecule that has been suggested to have a single π-bond, 1,2,2,3,4,4-hexa-tert-butylbicyclo[1.1.0]tetrasilane. I showed that while formation of a σ-bond is not expected, a weak multicenter σ-bond indeed forms in 4-membered 1,3- diradicals.
The origin of σ-bonding is a delocalized four-center σ-bond that is a low energy HOMO in the 4-membered ring. It is worth noting that although the single π-bond does not form in 1,3 diradicals, a unique bond with weak σ- and strong π-components form in the studied system. Manipulation of the population of the molecular orbitals provides numerical data that suggest the σ-bond is responsible for about 30% of the bonding while the π-bond contribution in total bonding is 70%.
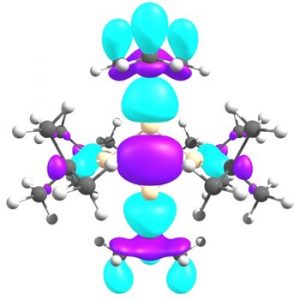
Figure: Low energy multicenter σ-bond forms as a result of the multicenter interaction in HOMO-6 of the molecule.
The article is published in Nature Communications: https://www.nature.com/articles/s41467-021-24238-x
Congratulations!