Article by the team of Prof. Daniel Gryko and Prof. Bartosz Grzybowski in Angewandte Chemie International Edition
Prof. Daniel Gryko in collaboration with both Prof. Bartosz Grzybowski (Institute for Basic Science, Ulsan, Korea) and Dr. Irena Deperasińska (Institute of Physics Polish Academy of Sciences) published the paper in Angewandte Chemie entitled: ‘The Hybrid of Indolizine and Merocyanine – A New Class of Organelle-specific Dyes’. Authors discovered a new class of dyes – indolizine-merocyanines (IndMer) and indolizine-cyanines – by unlocking the potential of electron-rich 2-hydroxyindolizines. Tandem Friedel-Crafts alkylation followed by intramolecular nucleophilic aromatic substitution afforded structurally diverse dyes, as both the nucleophilic and electrophilic partners can be broadly modified. A convergent fragment coupling strategy allowed rapid access to these π-conjugated merocyanines in three steps from pyridines. Uniform distribution of the HOMO and LUMO combined with negligible change of dipole moment upon excitation is responsible for the intense orange or red emission of this new family of reasonably photostable dyes in broad range of solvents. The new merocyanine dyes have the potential to target a variety of organelles – both uncharged and positively charged indolizine-merocyanines localize a subset of cellular lysosomes, positively charged indolizine-cyanine hybrid accumulates in mitochondria, while coumarin-merocyanine shows context-dependent localization to mitochondria and RNA-rich nucleoli of the living cells.
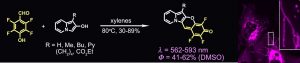
J. S. Araujo Badaro, B. Koszarna, M. Perkowska, K. Kandere-Grzybowska, D. V. Kolygina, O. Morawski, J. Park, B. Grzybowski, I. Deperasińska, D. T. Gryko, „Hybrid of Indolizine and Merocyanine—A New Class of Organelle-Specific Dyes”, Angew. Chem. Int. Ed.